- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
Featured Products
About Us
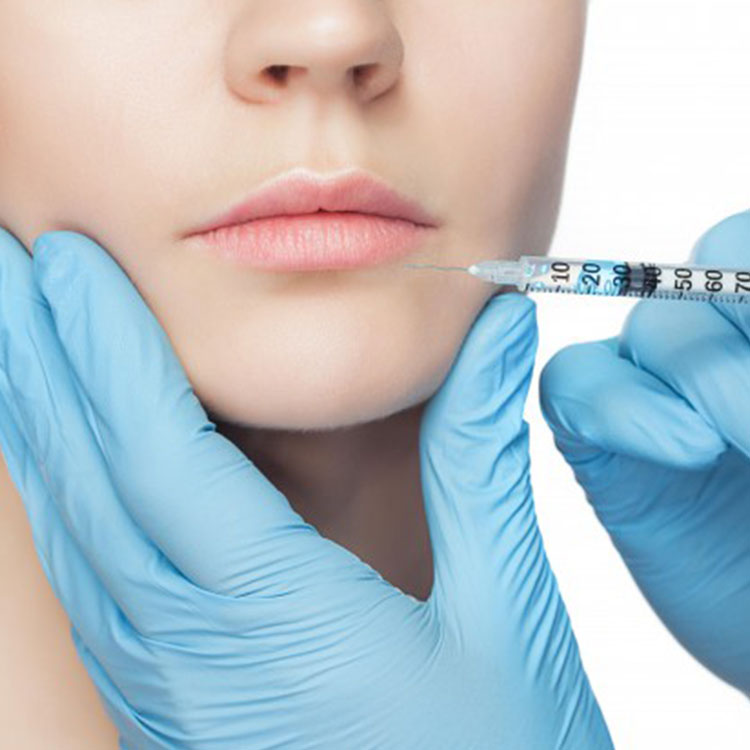
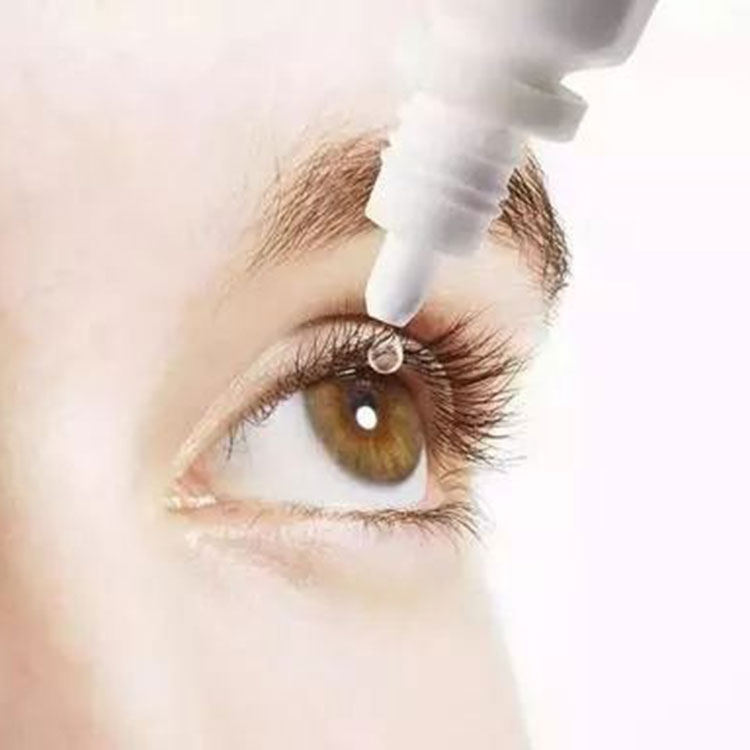
It has developed into a leading global supplier of hyaluronic acid relying on its technological innovation and superior product quality. It was also awarded the honorary titles of high-tech enterprise, enterprises with specialization, refinement, specialization and novelty, gazelle enterprise and technology-based enterprise.
New Products
News

Amhwa Biopharm Co., Ltd. passed the US FDA inspection without defects!

Good news! Amhwa Biology ProEnzy ™ enzyme cutting technology obtained the national invention patent
Recently, the ProEnzy™ enzymatic digestion technology for the preparation of small molecule sodium hyaluronate, namely "a method for the preparation of small molecule hyaluronate or its salt", was declared by Amhwa Biology. It has been officially authorized by the State Intellectual Property Office and granted the certificate of Chinese Invention patent, which is a breakthrough in the field of biological engineering technology in our country.

Good news! Amhwa Biology ProEnzy ™ enzyme cutting technology obtained the national invention patent
Recently, the ProEnzy™ enzymatic digestion technology for the preparation of small molecule sodium hyaluronate, namely "a method for the preparation of small molecule hyaluronate or its salt", was declared by Amhwa Biology. It has been officially authorized by the State Intellectual Property Office and granted the certificate of Chinese Invention patent, which is a breakthrough in the field of biological engineering technology in our country.

Amhwa Biology export qualification of EU API approved
Recently, after the review of the Shandong Provincial Drug Administration, Amhwa Biology officially approved the qualification of the Export of raw material drugs to the EU. As the second API supplier in China to master the extraction of sodium hyaluronate by biological fermentation, this approval marks the official entry of Amhwa Biology ProHA ® sodium hyaluronate API into the EU market.

On May 19, 2017, Amhwa Biology received the DMF filing Registration Number confirmation letter from the US FDA
On May 19, 2017, Amhwa Biology received the DMF filing Registration Number confirmation letter from the US FDA, informing Amhwa Biology that it had obtained the DMF filing registration number 031799.

Amhwa Biology presents pharmaceutical grade products at the European Pharmaceutical Raw Materials Exhibition Trip to Barcelona
CPHI Barcelona was successfully held in Fira Barcelona Gran Via, Spain from 24 to 26 October 2023. As a high-profile global pharmaceutical event, this year's event builds on a legacy of excellence by bringing together suppliers, innovators and pharmaceutical professionals in a unique way, combining the best of both offline and online experiences.