- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
Medical Grade Sodium Hyaluronate
AMHWA® is a professional leader China Medical Grade Sodium Hyaluronate manufacturer with high quality and reasonable price. Welcome to contact us. Sodium hyaluronate, also known as hyaluronan, or HA, is a natural polysaccharide existing in human and animal bodies, mainly in the soft connective tissues such as the skin, vitreous humor of eyes and synovial fluid of joints.
Send Inquiry
Medical Grade Sodium Hyaluronate
AMHWA® is a professional Medical Grade Sodium Hyaluronate manufacturers and suppliers in China, you can rest assured to wholesale and customized Medical Grade Sodium Hyaluronate from our factory and we will offer you the best after-sale service and timely delivery.
Chemical Formula: (C14H20NNaO11)n
Cas: 9067-32-7
Source: Microbial fermentation

Sodium hyaluronate, also known as hyaluronan, or HA, is a natural polysaccharide existing in human and animal bodies, mainly in the soft connective tissues such as the skin, vitreous humor of eyes and synovial fluid of joints. HA is a high molecular weight mucopolysaccharide composed of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine. Its random coiled configuration and fluid kinetics characteristic in solutions gives it some important physical characters like moisturizing and lubricating properties, viscoelasticity and pseudo-plasticity and also because of its good biocompatibility, it is widely used in pharmaceuticals and medical devices.
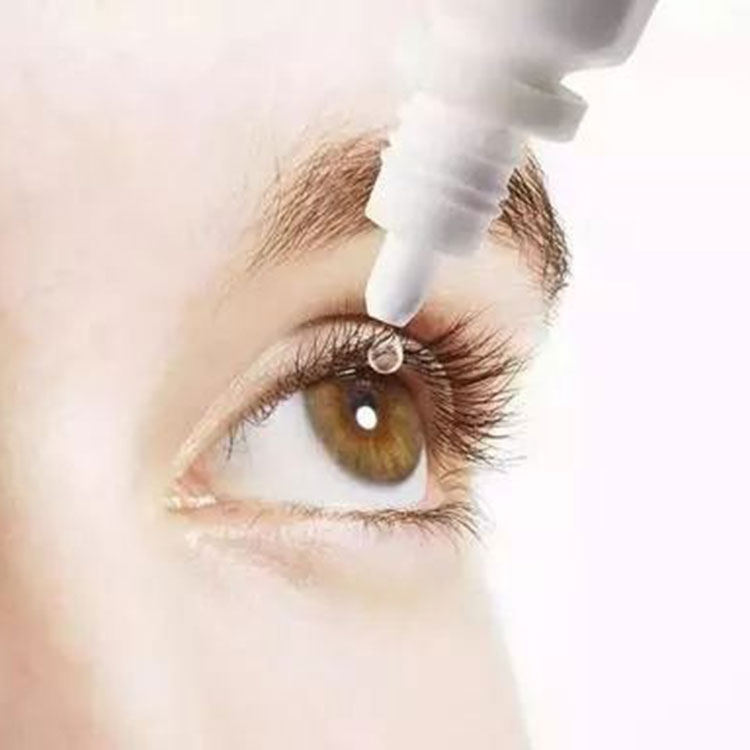
EvoHA™ is a pharmaceutical grade sodium hyaluronate which can be used as an API or excipient for drugs and medical devices in ophthalmic preparations, intra-articular injections, anti-adhesive preparations, topical preparations for wound healing and soft tissue filler, etc. sodium hyaluronate is divided into two categories based on the applications, medical grade and injection grade.
Functions & Applications
| Product | Functions | Applications |
| Medical Grade | Lubricating, moisturizing, improving efficacy of drugs, relieving dry eye syndrome, and promote healing of corneal and conjunctival injury | Eye drops, eye lotions, contact lens solutions, medical lubricants, etc. |
| Wound healing | Topical preparations (formulations of gel, film, etc.) | |
| Drug or cell carrier / matrix | Eye drops, cell culture, etc. | |
| Repairing damaged mucous membrane or cartilage, etc. | Oral pharmaceutical preparations |
Advantage
GMP / WC-GMP Certified
CEP/EDQM & DMF Certified
ISO 9001 / ISO 13485
CDE Registered: Y20200000498 (A)
High Purity
ICH Q7
Non-GMO Bacterial fermentation
High Purity, Low Bacterial Endotoxin
GMP / cGMP / ICH Q7 / CEP / FDA / CE / DMF / NMPA / ISO 13485 / ISO 9001